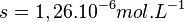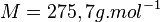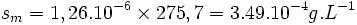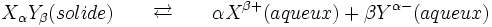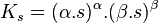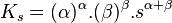an introduction
The dissolution product It is the equilibrium constant corresponding to the dissociation of a solid in a solvent.
identification
Consider for example the dissociation of an ionic solid of formula Xαsβ
The dissolution is described by the following reaction:
Employment law use Mass (The term mass is used to designate two quantities connected to one…) We get the formula:
- to mex : Activity (The term activity can refer to a profession.) From’Ocean (In the life sciences, species (from the Latin species, ‘kind’…) x
Being an ionic compound as a pure solid, its activity is equal to 1. The activities of ions in an aqueous medium are assimilated to their concentrations in moles by Liter (The liter (from Greek λίτρα lítra, an ancient measure of capacity…) (mol/L).
The dissolution product (The product of solubility is the equilibrium constant corresponding to solubility…) he is :

If the product concentrations ( [X+].[Y–] ) of the ions constituting the ionic compound remains less than KsThe ionic compound dissociates completely.
If the product [X+].[Y–] The solution is reached saturated andaddition (Addition is an elementary operation that allows in particular to describe …) of ionic compounds resulting in a precipitate (In chemistry and mineralogy, a precipitate is a phase formation…). Additional addition of compound XY does not modify the concentration of the ions but increases the concentration amount (quantity is a general term for measurement (compute, amount); numerical, …) from the precipitate.
The relationship between solubility product and solubility
|
be careful The relationships and methods of calculation provided for in this paragraph apply only in the event of termination for a single ionic compound : If other elements already exist or have been added, they must be taken into account. |
An example of an ionic compound of type XY
bromide copper (Copper is a chemical element with symbol Cu and atomic number 29. Cu…) dissolves inWater (Water is a chemical compound found everywhere on Earth, essential for everyone…) According to the following scale:
Let it be soluble in copper bromide. Dissolving a mole per liter of CuBr gives one mole per liter of Cu+ and s mol per liter of Br–. we can describe Situation (In geography, position is a spatial concept that allows for the relative location of…) in the following way:
 |
|||
| chemical species | CuBr | copper+ | Br– |
|---|---|---|---|
| t = 0 | s | 0 | 0 |
| balance | 0 | s | s |
The solubility product of copper bromide is written:
CAUTION: If the equilibrium constant is dimensionless, it must be strictly balanced by multiplying or dividing as necessary by the scalar concentration against0 = 1MOhI.The -1 This is it :
In general we neglect this term in C° to retain the first write, even if the dimensional equation is wrong.
So
The molar mass of copper bromide is
MagainstaBs = 63.55 + 79.90 = 143.45g.MOhI -1And the
The total solubility of copper bromide is
An example of an ionic compound of type X2s
carbonatesilver (Silver metal or silver is a chemical element with the symbol Ag – du…) Dissolves in balance:
Let’s melt silver carbonate. Mole dissolution of AgCO3 It gives 2s mole of Ag+ and s is a mole of carbon monoxide3–. The situation can be described as follows:
 |
|||
| chemical species | AG2co3 | AG+ | co32- |
|---|---|---|---|
| t = 0 | s | 0 | 0 |
| balance | 0 | 2 sec | s |
So
The molar mass of silver carbonate is
Comprehensive dissolution of silver carbonate
Generalization (Generalization is the process of abstracting out a set of…)
Consider the dissolution of an ionic compound of general formula Xαsβ
Let’s melt Xαsβ. Mol X dissolutionαsβ It gives the α mole of Xα and β mole of Yβ…the situation can be described as follows:
 |
|||
| chemical species | Xαsβ | Xβ+ | sα- |
|---|---|---|---|
| t = 0 | s | 0 | 0 |
| balance | 0 | αs | βs |
The general relationship between K_s and solubility is as follows:

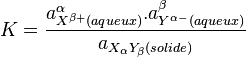

![a_{X^{\beta +}(aqueous)}^{\alpha } = \left[ X^{\beta+} \right] ^{\alpha}](https://www.techno-science.net/illustration/Definition/inconnu/5/53f84df4672e9f5ccbc5acada51f1e0d.png)
![a_{Y^{\alpha-}(aqueous)}^{\beta } = \left[ Y^{\alpha-} \right] ^{\beta}](https://www.techno-science.net/illustration/Definition/inconnu/8/849ee04092d168e5dce9c50ad6b0aa52.png)
![K_s = \left[ X^{\beta+} \right] ^{\alpha}. \the left[ Y^{\alpha-} \right] ^{\beta}](https://www.techno-science.net/illustration/Definition/inconnu/9/9ae67384ed649e2629a7833b48415ed4.png)

![K_s = \left[ Cu^+ \right] . \the left[ Br^- \right] = 5.3. 10^{-9}](https://www.techno-science.net/illustration/Definition/inconnu/b/b5d465d65e13c9c220facabaa6a57520.png)
![K_s = \frac{\left[ Cu^+ \right] . \the left[ Br^- \right]} {{C^0}^2} = 5.3. 10^{-9}](https://www.techno-science.net/illustration/Definition/inconnu/3/3cad1e861212f8a9c17b8aa26e6dea64.png)
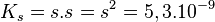
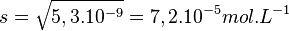
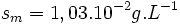
![K_s = \left[ Ag^+ \right]^ 2. \left[ CO_3^{2-} \right] = 8,1.10^{-18}](https://www.techno-science.net/illustration/Definition/inconnu/0/08edb33b7ee2b674d752523d4160fad4.png)
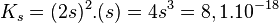
![s = \sq[3]{8,1.10^{-18}/4}](https://www.techno-science.net/illustration/Definition/inconnu/7/72affc1aa40658eda885783098341d64.png)